Snowmaking Science
Two of the Most Important Variables in Making Snow are:
- Temperature & Humidity – The relationship between temperature and humidity is called Wet bulb Temperature.
Both Temperature and Humidity must be low enough for Snowmaking. Click here to find a live snowmaking weather tool that will give you the current snowmaking conditions where you live. This tool will also give you your snowmaking forecast and snowmaking history (how many days you could have made snow last year). On the weather tool page you will also find a snowmaking weather chart you can print out to determine if the temperature and humidity are right for you to make snow. This chart also shows Wet bulb temperature. (definition found below) - Water temperature, very simply put the colder the better. Commercial snow makers at ski areas typically use water from ponds, this water temperature is usually from 34 to 40 deg. Fahrenheit. Our Snowmakers are designed specifically to be used with household water supply which is typically 15 to 25 deg. Fahrenheit warmer then commercial water supply for ski areas.
How does it work? (The Quick Explanation):
The science of snowmaking can be quite complex. For the majority of us, however, a simple explanation of how the different parts of a snowmaker act will suffice. Snowmaking in its simplest form is the act of turning water into small ice crystals (snow). In order to make snow from your home water supply (at about 50°F), it must be cooled very rapidly. Four things come into play to make this happen: ambient temperatures, evaporation, surface area, super cooling.
Ambient Temperature
First it must be cold outside. Even when the outdoor temperature is below freezing (32°F) snow quality can be poor or slushy. This is because much of the water is not staying or even turning into the frozen state. If you refer to our snowmaking weather chart, you will see what the ideal temperatures are for snow making.
Evaporation
The second factor is heat loss through evaporation. As some of the water evaporates from the surface of the drop a small amount of heat is removed from the drop itself. Try putting some rubbing alcohol on your arm. As it evaporates you will experience this cooling effect. Your body uses this process of evaporation to cool itself, we call it sweating. When the air is humid, there is already a lot of moisture in the air. Your sweat is less readily absorbed into the air and is unable to evaporate from your skin removing the heat with it. The same premise happens in snowmaking. When there is high humidity, the water droplet’s surface is not able to evaporate a small amount of water and remove some of the heat. Therefore, in snowmaking we must refer to the “Wet Bulb Temperature”. This is a measure of the ambient temperature that takes into account the cooling effect the humidity in the air allows for.
Surface Area
The third way we cool the water is by increasing the surface area of the drop. By increasing the surface area, we expose as much of the water to the cold as possible. The smaller we make these drops, the greater the surface area to volume ratio. We achieve the proper drop size and spray pattern through our highly specialized nozzles. And yes, the nozzles do matter! In order to optimize the size of the droplets, the distance between the drops, and the water volume flowing though the opening while employing high pressures to achieve proper distance and hang time, we engineered nozzles specifically for snowmaking.
Super Cooling
Finally we need to look at super cooling. When a compressed gas (in this case air) is allowed to rapidly expand, there is a decrease in temperature. This is known as the Joule-Thomson Effect. The conditions at the air nozzle are such that the mist coming from the nucleation nozzle is able to immediately freeze. These tiny ice crystals are then drafted into the larger upper mists which seed and snap the pre-cooled water droplets into a frozen state. The result is snow that then falls out of the mist.
The Science of Snow Making by:

Heat Exchange Process
Snowmaking is a heat exchange process. Heat is removed from snowmaking water by evaporative and convective cooling and released into the surrounding environment. This heat creates a micro-climate inside the snowmaking plume that is very different from ambient conditions. Understanding this process can lead to practical benefits to the snowmaker.
There are many variables that affect snowmaking. Three of the most important variables are wet bulb temperature, nucleation temperature and droplet size.
Wet Bulb Temperature
The temperature of a water droplet exiting a snow gun is typically between 34 F and 44 F. Once a water droplet passes the nozzle and is released into the air, its temperature falls rapidly due to expansive and convective cooling and evaporative effects. The droplet’s temperature will continue to fall until equilibrium is reached. this is the wet bulb temperature and it is as important as dry bulb (ambient) temperature in predicting snowmaking success. For example, snowmaking temperatures at 28 F and 10% humidity are equivalent to those at 20 F and 90% humidity.
Nucleation Temperature
Once the wet bulb temperature is know, there must be a way to predict whether water droplets will actually freeze at that temperature.
Ice is the result of a liquid (water) becoming a solid (ice) by an event called nucleation. A water droplet must first reach its nucleation temperature to freeze. there are two types of nucleation, homogeneous nucleation and heterogeneous nucleation.
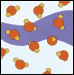
Water Molecules in Liquid Form
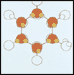
(Solid Form) Molecules Form A Hexagonal Array
Homogeneous Nucleation
Homogeneous nucleation occurs in pure water with no contact with any other foreign substance or surface. With homogeneous nucleation, the conversion of the liquid state to solid state is done by either lowering temperatures or by changes in pressure. However, the primary influence on the conversion of water to ice or ice to water is temperature.
In homogeneous nucleation, the nucleation begins when a very small volume of water molecules reaches the solid state. This small volume of water molecules reaches the solid state. This small volume of molecules is called the embryo and becomes the basis for further growth until all of the water is converted. the growth process is controlled by the rate of removal of the latent heat being released. Molecules are attaching and detaching from the embryo at roughly equal and very rapid rates. As more molecules attach to the embryo, energy is released causing the temperature of the attached molecules to be lower than the temperature of the unattached molecules. The growth rate continues until all the molecules are attached. At this point, you have the solid state (ice).
Most of us would think pure water freezes at 0 C or 32 F. In fact, the nucleation event (freezing) for pure water will take place as low as minus 40 C or minus 40 F. This is mostly likely to occur in laboratory experiments or high in the upper atmosphere (upper troposphere).
Heterogeneous Nucleation
The heterogeneous nucleation process is when ice forms at temperatures above minus 40 C or minus 40 F due to the presence of a foreign material in the water. this foreign material acts as the embryo and grows more rapidly than embryos of pure water. The location on which an ice embryo is formed is called an ice-nucleating site. As with homogeneous nucleation, heterogeneous nucleation is governed by two major factors: the free energy change involved in forming the embryo and the dynamic of fluctuating embryo growth. In heterogeneous nucleation, the configuration and energy of interaction at the nucleating site become the dominating influence in the conversion of water to ice. Snowmaking involves the process of heterogeneous nucleation.
There are many materials and substances which act as nucleates, each promotes freezing at a specific temperature or nucleation temperature. they are generally categorized as high-temperature (i.e., sliver iodide, dry ice and ice nucleating proteins) or low-temperature (i.e., calcium, magnesium, dust and silt) nucleates. It is the low-temperature nucleators that are found in large numbers in untreated snowmaking water. the nucleation temperature of snowmaking water is between 15 F and 20F.
But wait a minute! Why do you hear freezing warnings at temperatures around 32 F? The answer is that another factor is coming into play with the freezing process. That factor is called surface (i.e., roads, highways, trees). There is an energy interaction between the ice-nucleating site in the water with the surface. This causes the water droplets to freeze very near 32 F or 0 C.
In snowmaking it is the nucleator having the highest nucleation temperature that determines when a water droplet will freeze.
As a water droplet cools, heat energy is released into the atmosphere at a rate of one calorie per gram of water. As it freezes into an ice crystal, the water droplet will release additional energy at a rate of 80 calories per gram of water. this quick release of energy raises the water droplet temperature to 32 F, where it will remain while freezing continues. This is one reason why we are accustomed to thinking that water freezes at 32 F. To be precise, the water will continue to freeze as long as it remains at or below 32 F, but only after it has first cooled to its nucleation temperature. Any excess energy will be dissipated into the atmosphere.
Droplet Size
Since the distribution of various nucleators in a given volume of water is totally random, the size of the water droplet or the number of high-temperature nucleators has a significant effect on the temperature at which freezing occurs (nucleation temperature). In natural water, as the size of the water droplet decrease, the likelihood that the droplet will contain a high-temperature nucleator also decreases. Conversely, larger water droplets stand a better chance of containing high-temperature nucleators. The optimum situation for snowmakers is one where each and every droplet of water passing through the snow gun nozzle contains at least one high-temperature nucleator and where each droplet freezes in the plume.
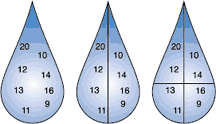
Numbers in the droplets represent various ice-nucleating sites with different nucleation temperatures. The highest number nucleator in the droplet will determine at what temperature the water droplet will freeze.
The relationship between the variables of nucleation temperature and droplet size is summarized in two statistically valid conclusions. Firstly, a 50% increase in the droplet size results in a one-degree F increase in nucleation temperature. Secondly, a 50% decrease in droplet size results in a three-degree F decrease in nucleation temperature. These conclusions are based on an average droplet size can by counter-productive to promoting high-temperature nucleation, unless enough high-temperature nucleators are present to compensate.
Looking at the relationship between droplet size and evaporation, research in cloud seeding show that:
These conclusions further point out the undesirable results from using very small droplets, especially in areas where water loss is a critical issue.
Relating droplets size to nucleation temperature, it is possible to increase snowmaking production and efficiency by using high-temperature nucleators together with larger water droplets. this frequently not only allows increased water flow, but also reduces evaporation and yields more snow on the ground. In fact, studies indicate that a 20% increase in water flow can increase snow volume up to 40% if droplet size and nucleation temperature is optimized.
In conclusion, a better understanding of the dynamics between wet bulb temperatures; nucleation temperature and droplet size together with a practical application of the science involved can help improve the efficiency of the snow manufacturing process.
Ready to Buy?
Order your snowmaker online or call us at 1-800-957-7128

